Overview of Acute Myeloid Leukaemia in Children
Acute myeloid leukaemia (AML) is a rapidly progressing cancer of the blood and bone marrow characterized by the uncontrolled growth of immature myeloid cells, called myeloblasts. These abnormal cells accumulate in the bone marrow, disrupting the production of normal blood cells and leading to anemia, infection, and bleeding disorders.
While AML is less common in children than acute lymphoblastic leukaemia (ALL), it accounts for about 15–20% of childhood leukaemia cases. It can occur at any age, including infancy, but is most often diagnosed in children under 2 and in teenagers. Due to advances in therapy, the long-term survival rate for pediatric AML has improved significantly and is now around 65–75% with current treatment protocols.
Commonly Associated With Acute Myeloid Leukaemia in Children
Several factors increase the risk of AML in children, including:
- Genetic syndromes: Down syndrome, Fanconi anemia, Bloom syndrome, and Li-Fraumeni syndrome.
- Previous cancer treatments: Prior chemotherapy or radiation therapy.
- Inherited predispositions: Familial platelet disorder with RUNX1 mutation, congenital neutropenia.
- Bone marrow disorders: Myelodysplastic syndromes (MDS) or myeloproliferative neoplasms (MPN).
- Environmental exposures: High-dose ionizing radiation and exposure to benzene (rare in children).
- Certain infections in utero and prenatal exposures are under study but not definitively linked.
Children with Down syndrome are at increased risk for a specific subtype known as acute megakaryoblastic leukaemia (AMKL).
Causes of Acute Myeloid Leukaemia in Children
The exact cause of AML in children is not fully understood, but it develops when genetic mutations occur in immature myeloid cells, causing them to multiply uncontrollably and fail to mature. These abnormal cells crowd the bone marrow and inhibit the production of healthy blood cells.
Key contributing factors include:
- Chromosomal translocations and gene mutations (e.g., t[8;21], inv[16], MLL rearrangements) that alter cell growth.
- Inherited genetic predisposition to hematologic malignancies.
- DNA damage from prior chemotherapy or radiation therapy.
- Environmental exposures that cause DNA mutations (rare in children).
- Spontaneous mutations that occur during fetal development or early life.
Symptoms of Acute Myeloid Leukaemia in Children
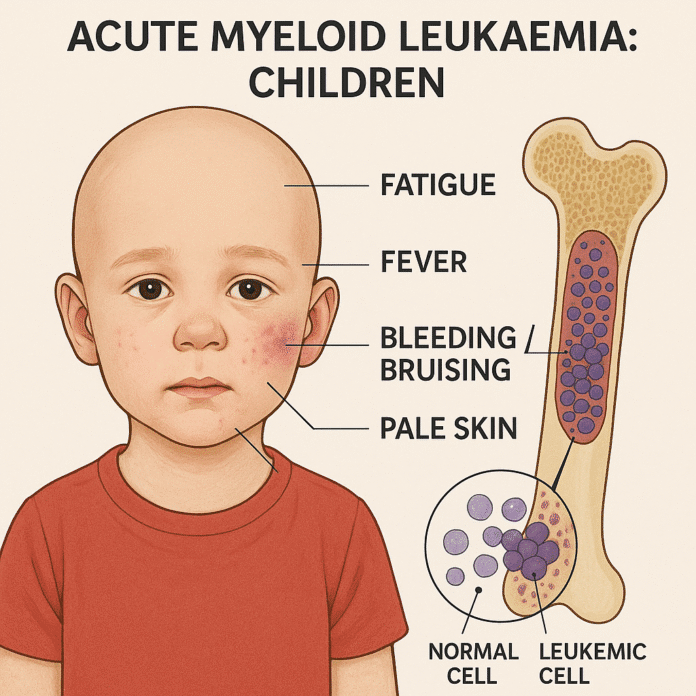
Symptoms of AML in children often develop quickly — over days or weeks — and reflect bone marrow failure and infiltration of leukemic cells into various organs. Common signs and symptoms include:
- Anemia symptoms: Fatigue, pale skin, shortness of breath.
- Increased infections: Frequent fevers and recurrent infections due to low white blood cell function.
- Bleeding problems: Easy bruising, petechiae, nosebleeds, bleeding gums due to low platelet counts.
- Bone and joint pain: Especially in the legs, hips, and back.
- Swollen gums (gingival hypertrophy): Particularly in monocytic subtypes.
- Swelling of lymph nodes, liver, or spleen.
- Unexplained weight loss, night sweats, and loss of appetite.
- Leukostasis symptoms: Headaches, confusion, or vision changes if white blood cell counts are extremely high.
Because AML progresses rapidly, early recognition and prompt medical attention are crucial.
Exams & Tests for Acute Myeloid Leukaemia in Children
Diagnosis of childhood AML involves blood tests, bone marrow analysis, and molecular studies:
- Complete blood count (CBC): Shows anemia, thrombocytopenia, and abnormal white blood cell counts.
- Peripheral blood smear: Reveals circulating myeloblasts, often with Auer rods — needle-like inclusions typical of AML.
- Bone marrow aspiration and biopsy: Confirms the diagnosis when ≥20% blasts are present.
- Immunophenotyping (flow cytometry): Identifies myeloid markers such as CD13, CD33, and MPO.
- Cytogenetic and molecular tests: Detect genetic mutations and chromosomal abnormalities important for risk stratification and treatment planning.
- Lumbar puncture: Performed if central nervous system involvement is suspected.
- Imaging (e.g., ultrasound, chest X-ray): May be used to assess organ infiltration or chloromas (myeloid sarcomas).
Treatment of Acute Myeloid Leukaemia in Children
Treatment for pediatric AML is intensive and must begin promptly after diagnosis. The main goals are to achieve remission, eradicate residual disease, and prevent relapse.
1. Induction Therapy
- Goal: Achieve remission by eliminating leukemic blasts from the bone marrow.
- Regimen: Combination chemotherapy (e.g., cytarabine, daunorubicin, etoposide).
- Success rate: About 85–90% of children achieve remission after induction.
2. Consolidation Therapy
- Goal: Eliminate any remaining leukemia cells and reduce relapse risk.
- Regimen: Additional cycles of intensive chemotherapy.
- Some children with high-risk disease may undergo allogeneic stem cell transplantation during this phase.
3. Targeted Therapies
- Used when specific genetic mutations are present:
- FLT3 inhibitors (e.g., midostaurin)
- IDH1/IDH2 inhibitors for rare mutations
- Gemtuzumab ozogamicin, a monoclonal antibody, is sometimes added for certain AML subtypes.
4. Supportive Care
- Blood transfusions and antibiotics to manage complications.
- Management of tumor lysis syndrome and leukostasis.
- Psychosocial and nutritional support throughout treatment.
5. Stem Cell Transplantation
- Considered for children with high-risk AML, relapsed disease, or poor response to chemotherapy.
With modern therapy, the overall survival rate is about 65–75%, and ongoing research continues to improve outcomes, especially for high-risk cases.
Source
- Creutzig U, et al. “Acute myeloid leukemia in children.” N Engl J Med 2012; 368(12):1204–1216.
- Meshinchi S, Arceci RJ. “Biology and treatment of pediatric acute myeloid leukemia.” J Clin Oncol 2017; 35(9):943–953.
- National Cancer Institute. “Childhood Acute Myeloid Leukemia Treatment (PDQ®) – Health Professional Version.” NCI, 2024.